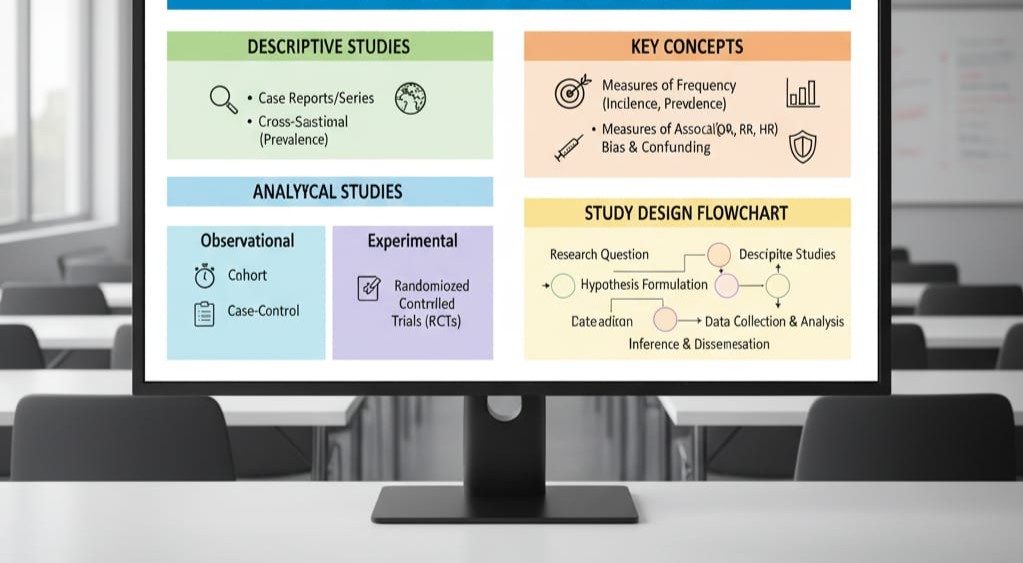
This intensive five-day training course is designed to equip participants with a solid foundation in Epidemiological Research Methods, focusing on the principles, study designs, and analytical techniques used to investigate health-related states or events in specified populations. The course moves beyond basic biostatistics to concentrate on the logic of causal inference, measurement of disease burden, and the critical evaluation of health research literature. It is an essential course for professionals dedicated to evidence-based public health practice, clinical research, and policy development.
The curriculum is structured across 10 progressive modules, covering the core stages of epidemiological inquiry. Key topics include measures of occurrence and association (e.g., incidence, prevalence, risk ratios), detailed analysis of major study designs (cohort, case-control, and trials), techniques for controlling bias and confounding, and introduction to regression modeling in epidemiology. The course also delves into public health surveillance, outbreak investigation, and the crucial elements of research ethics and proposal development, ensuring participants can competently plan, execute, and interpret epidemiological studies.
Who should attend the training
Objectives of the training
Course Duration: 5 days
Training fee: USD 1500
Training methodology
Trainer Experience
Our trainers are seasoned epidemiologists and public health researchers, holding advanced degrees and having extensive experience in academic research, government public health agencies, and international organizations. They possess specialized expertise in study design, biostatistics, and translational research.
Quality Statement
We are committed to delivering a high-quality, scientifically rigorous, and highly practical training program in Epidemiological Research Methods. Our curriculum is designed to ensure participants not only grasp the theoretical foundations but can immediately and competently apply these methods to complex health issues.
Tailor-made courses
This course can be customized to focus on specific sub-disciplines of epidemiology (e.g., chronic disease, infectious disease, environmental), concentrate on a particular statistical software package, or integrate your organization's proprietary public health data and challenges for case study application. We offer flexible delivery options, including on-site, virtual, and blended learning solutions to meet your organizational needs.
Requirements:
Terms and Conditions
1. Discounts: Organizations sponsoring Four Participants will have the 5th attend Free
2. What is catered for by the Course Fees: Fees cater for all requirements for the training – Learning materials, Lunches, Teas, Snacks and Certification. All participants will additionally cater for their travel and accommodation expenses, visa application, insurance, and other personal expenses.
3. Certificate Awarded: Participants are awarded Certificates of Participation at the end of the training.
4. The program content shown here is for guidance purposes only. Our continuous course improvement process may lead to changes in topics and course structure.
5. Approval of Course: Our Programs are NITA Approved. Participating organizations can therefore claim reimbursement on fees paid in accordance with NITA Rules.
Booking for Training
Simply send an email to the Training Officer on training@armstrongglobalinstitute.com and we will send you a registration form. We advise you to book early to avoid missing a seat to this training.
Or call us on +254720272325 / +254725012095 / +254724452588
Payment Options
We provide 3 payment options, choose one for your convenience, and kindly make payments at least 5 days before the Training start date to reserve your seat:
1. Groups of 5 People and Above – Cheque Payments to: Armstrong Global Training & Development Center Limited should be paid in advance, 5 days to the training.
2. Invoice: We can send a bill directly to you or your company.
3. Deposit directly into Bank Account (Account details provided upon request)
Cancellation Policy
1. Payment for all courses includes a registration fee, which is non-refundable, and equals 15% of the total sum of the course fee.
2. Participants may cancel attendance 14 days or more prior to the training commencement date.
3. No refunds will be made 14 days or less before the training commencement date. However, participants who are unable to attend may opt to attend a similar training course at a later date or send a substitute participant provided the participation criteria have been met.
Tailor Made Courses
This training course can also be customized for your institution upon request for a minimum of 5 participants. You can have it conducted at our Training Centre or at a convenient location. For further inquiries, please contact us on Tel: +254720272325 / +254725012095 / +254724452588 or Email training@armstrongglobalinstitute.com
Accommodation and Airport Transfer
Accommodation and Airport Transfer is arranged upon request and at extra cost. For reservations contact the Training Officer on Email: training@armstrongglobalinstitute.com or on Tel: +254720272325 / +254725012095 / +254724452588
| Course Dates | Venue | Fees | Enroll |
|---|---|---|---|
| Aug 03 - Aug 07 2026 | Zoom | $1,300 |
|
| Sep 07 - Sep 11 2026 | Nairobi | $1,500 |
|
| May 04 - May 08 2026 | Nakuru | $1,500 |
|
| Jul 20 - Jul 24 2026 | Nanyuki | $1,500 |
|
| Jul 06 - Jul 10 2026 | Naivasha | $1,500 |
|
| Jul 06 - Jul 10 2026 | Mombasa | $1,500 |
|
| Jul 13 - Jul 17 2026 | Kisumu | $1,500 |
|
| Jun 01 - Jun 05 2026 | Kigali | $2,500 |
|
| Jun 08 - Jun 12 2026 | Kampala | $2,500 |
|
| Jul 06 - Jul 10 2026 | Arusha | $2,500 |
|
| May 04 - May 08 2026 | Johannesburg | $4,500 |
|
| Jun 01 - Jun 05 2026 | Marrakesh | $4,500 |
|
| Aug 03 - Aug 07 2026 | Victoria | $4,500 |
|
| Jun 01 - Jun 05 2026 | Pretoria | $4,500 |
|
| Jul 13 - Jul 17 2026 | Cairo | $1,500 |
|
| Mar 02 - Mar 06 2026 | Dubai | $5,000 |
|
| Jun 08 - Jun 12 2026 | Riyadh | $5,000 |
|
| Oct 05 - Oct 09 2026 | Doha | $5,000 |
|
| Jun 22 - Jun 26 2026 | London | $6,500 |
|
| Oct 12 - Oct 16 2026 | Paris | $6,500 |
|
| Jul 20 - Jul 24 2026 | Berlin | $6,500 |
|
| May 11 - May 15 2026 | Zurich | $6,500 |
|
| Jun 01 - Jun 05 2026 | New York | $6,950 |
|
| May 18 - May 22 2026 | Los Angeles | $6,950 |
|
| Sep 14 - Sep 18 2026 | Washington DC | $6,950 |
|
| May 04 - May 08 2026 | Toronto | $7,000 |
|
| Aug 03 - Aug 07 2026 | Vancouver | $7,000 |
|

Armstrong Global Institute
Typically replies in minutes